Pulvinic acid
| |
| Names | |
|---|---|
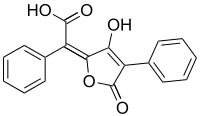
| IUPAC name
(2E)-(5-Hydroxy-3-oxo-4-phenyl-2(3H)-furanylidene)(phenyl)acetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H12O5 | |
| Molar mass | 308.289 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pulvinic acids are natural chemical pigments found in some lichens,[1] derived biosynthetically from the aromatic amino acids phenylalanine and tyrosine, via dimerization and oxidative ring-cleavage of arylpyruvic acids, a process that also produces the related pulvinones.[2][page range too broad]
Hydroxypulvinic acid pigments (pulvinic acid type family of pigments) have been found in Boletus (e.g. Boletus erythropus), Boletinus, Chalciporus, Gyrodon, Leccinum, Pulveroboletus, Suillus (e.g. Suillus luteus, Suillus bovinus, and Suillus grevillei), Paxillus (e.g. Paxillus involutus), Serpula (e.g. Serpula lacrymans), Xerocomus (e.g. Xerocomus chrysenteron), Hygrophoropsis (e.g. Hygrophoropsis aurantiaca), Retiboletus (e.g. Retiboletus nigerrimus), Pulveroboletus (e.g. Pulveroboletus auriflammeus), and are generally characteristic of Boletales.[3][2] In addition to pulvinone, derivatives and related pigments of this family include atromentic acid, xerocomic acid, isoxerocomic acid, variegatic acid, variegatorubin, xerocomorubin, chinomethide, methyl bovinate, badion A, norbadion A, bisnorbadiochinone A, pisochinone, and sclerocitrin.[3][2] More complex dimers of the pulvinic acid dimer (dimers of dimers) have been found in the fungi Scleroderma citrinum and Chalciporus piperatus.[4]
References
[edit]- ^ Bourdreux, Yann; Bodio, Ewen; Willis, Catherine; Billaud, Célia; Le Gall, Thierry; Mioskowski, Charles (2008). "Synthesis of vulpinic and pulvinic acids from tetronic acid". Tetrahedron. 64 (37): 8930–8937. doi:10.1016/j.tet.2008.06.058.
- ^ a b c Gill, M. & Steglich, W. (1987). "Pigments of Fungi (Macromycetes)". Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. Vol. 51. pp. 1–297. doi:10.1007/978-3-7091-6971-1_1. ISBN 978-3-7091-7456-2. PMID 3315906.
{{cite book}}:|journal=ignored (help)CS1 maint: multiple names: authors list (link)[page range too broad] - ^ a b "Isolierung und Strukturaufklärung von chemotaxonomisch relevanten Sekundärmetaboliten aus höheren Pilzen, insbesondere aus der Ordnung der Boletales" (PDF). Retrieved 2020-01-04.
- ^ Winner M, Giménez A, Schmidt H, Sontag B, Steffan B, Steglich W (2004). "Unusual pulvinic acid dimers from the common fungi Scleroderma citrinum (common earthball) and Chalciporus piperatus (peppery bolete)". Angewandte Chemie. 43 (14): 1883–6. doi:10.1002/anie.200352529. PMID 15054803.
